*(Adaptive Radiotherapy)
People are the worst, right? Humans have their own internal biases, their own ways of doing things, their own subjectivity and their own distractions.
So why don’t we just automate all treatment planning? This was certainly the attitude that I took at before beginning this placement in radiotherapy. It turns out that it’s not as simple as I expected…. What a surprise!
My first impressions of treatment planning were that it is definitely more of an art form than a science. Contouring organs is not in the skill set gained from 5 years + physics higher education. But once you learn where the rectum starts and ends (ischial tuberosities anyone?) and get over the fact that the bowel seems to occupy some people’s entire abdominal cavity, you can be safe in the knowledge that you’ve tried your hardest and that someone with years of study of anatomy behind them will (hopefully) be giving it the once-over.
Autocontouring
Auto segmentation and autocontouring organs at risk (OARs) using AI is now a thing and is even used clinically in 2 hospitals in Finland for prostate plans. MVision (https://mvision.ai/) is a cloud based system which sends anonymised patient scans (CT and/or MRI) outside the hospital and then uses its neural net trained from patient data from 6 partners in Finland, Estonia and Singapore to produce OAR outlines based on ESTRO guidelines. The prostate model is now CE marked for clinical use in Europe. Hosting it on the cloud means that it can be easily updated as the model learns more however does beg the question how to commission a system that is constantly evolving?
(I also have a lot of questions in terms of consent and personal data but we’ll save those for another day).
So say we do use autocontouring within the treatment planning pathway and no longer have to spend minutes (experienced people) or hours (me trying to contour bowel) tediously drawing around bits of anatomy. What can we do with this freed up time!? And more importantly, how can we get even more free time to work on novel techniques? What else could we automate?!
Automated Treatment Planning
Physicists and dosimetrists have already automated treatment planning to an extent. In each department there is usually a “recipe book” to follow for specific sites and dose prescriptions such that the planner has a starting point for beam angles etc rather than starting from scratch. But entire automation of the treatment planning seems like a pipe dream? Or is it?
Dose Prediction Knowledge based planning
Unsurprisingly, knowledge based planning uses prior experience to either predict an achievable dose or to give a better starting point for the planner to use. There are 2 separate methods:
Atlas based
Atlas based knowledge based planning selects the closest matching patients to build a predictive DVH model for the patient in question. This DVH however is only for the regions of interest (ROI) delineated, not other tissue so there’s no avoidance of hotspots etc, and no spatial information.
Model based
Model based knowledge based planning builds a model to predict the dose to individual voxels within the patient’s image.
Both of these methods mean that created plans can only be as good as the training data and that created plans can be clinically acceptable but not necessarily optimal. One advantage of this method is that the model is trained on patient anatomy and not treatment technique so transferring between treatment techniques should not be too difficult however the model must be trained for each clinical site.
As a tool for checking, predicting achievable dosimetry, training new staff, improving human planning and QA-ing clinical trials, this technique holds a lot of promise.
Protocol Based Automatic Iterative Optimisation (PB-AIO)
PB-AIO is an automated iterative adjustment of objective and constraints. A user defined template with required clinical objectives (hard constraints) is used to start with. AI systems can simulate the reasoning behaviour of a human planner, using “fuzzy logic” to convert trial and error of expert human planners to binary “IF-THEN” logic statements.
This leads to a reduction in inter-operator variability. Harder dose constraints are met at a similar level to human planning but the PB-AIO meets the less tight constraints more admirably than the humans. This is probably because a human operator’s focus is upon meeting the harder, tighter constraints.
This tool could be used to improve plan quality but not remove optimisation. Some experienced planners perform better than PB-AIO in particular cases.
Multicriteria Optimisation (MCO)
The pareto optimal solution in multicriteria optimisation is a solution that can’t be improved without degrading at least one other objective. Each pareto optimal solution is not necessarily clinical optimal but the clinically optimal plan will be a pareto optimal solution. There are an infinite number of pareto optimal solutions.
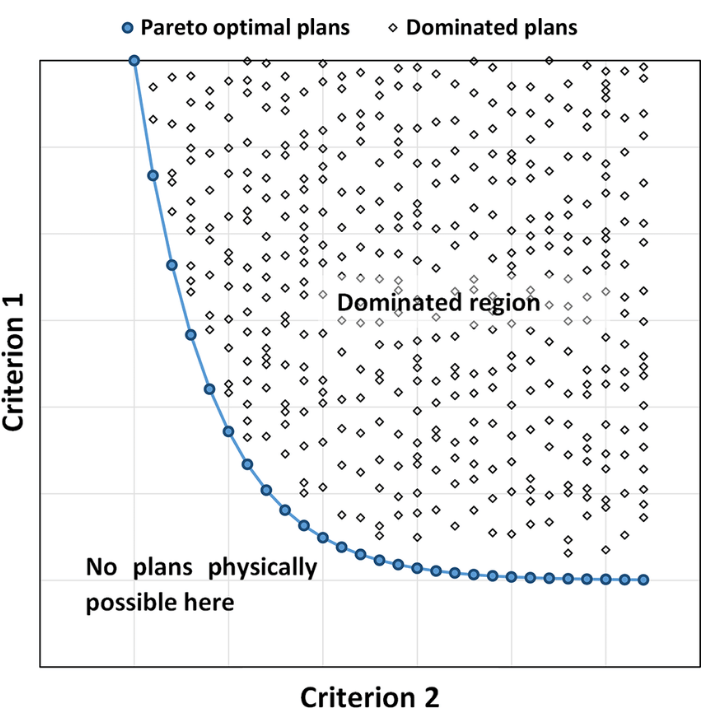
Automation in intensity-modulated radiotherapy treatment planning – a review of recent innovations – Scientific Figure on ResearchGate. Available from: https://www.researchgate.net/figure/Schematic-diagram-of-two-competing-criteria-The-graph-shows-a-large-number-of-different_fig2_326823269 [accessed 13 Jun, 2019]
A posteriori
Multiple plans are generated, each is a pareto optimal solution. Each is optimised to an extent where it can’t be improved upon without affecting other criterion. Each plan is a pareto optimal solution. An infinite number of plans could be generated but it is recommended N+1 plans are optimised where N= no. of objectives.
The human planner then reviews the N+1 plans produced and picks the clinically optimal solution.
These plans are optimised in fluence space which does not necessarily directly translate to machine parameter optimisation. The dosimetric difference is unlikely to be large but it can be especially in the case of very complex MLC movement patterns.
A priori
In “A priori MCO”, a single pareto optimal plan is generated by using a treatment site specific wishlist with assigned priorities and hard constraints. This multidisciplinary wishlist of criteria is made, reviewing recently treated patients and clinical protocols. Iterative improves of the wishlist will have to occur. These type of plans however do not allow for patient specific adaptation (e.g. prosthetic hip).
In 2 prospective studies, the a priori automatically generated plan was picked 32/33 and 29/30 times which shows strong promise!
Final thoughts
Automated treatment planning reduces variability between plans and could be used to prevent human bias and subjectivity when comparing different treatment techniques in a treatment planning study or when making the decision to support novel techniques. It could also be very valuable in using for “plan of the day” adaptive radiotherapy.
I don’t think all the humans are going to be out of a job just yet however. Humans perform better in unusual cases, and how do you get that experience? Years of planning!
Also who is going to make the plans to feed into the systems to train them? Who will help set wishlists for MCO? Who is going to pick the best plans? Who is going to check the plans are physically deliverable?
The final thing to note is that radiotherapy is done to improve a patient’s life. Our jobs are all about the patient coming first. Whether the radiotherapy treatment is palliative or curative, a clinician has taken the view that radiotherapy is a valuable part of the patient’s treatment. The above planning techniques are all linked to DVH-like metrics rather than patient outcomes so for radiotherapy to advance to being truly personalised, there is still some work to do.
10/10 can recommend a read of this review article if you’re interested: https://www.birpublications.org/doi/10.1259/bjr.20180270